 ,
Ismael Gastón Castillo Cortéz2
,
Ismael Gastón Castillo Cortéz2  ,
Isnel Martínez Montenegro3
,
Isnel Martínez Montenegro3  ,
O. Lorena Ibañez San Martín2
,
O. Lorena Ibañez San Martín2 
Introduction. The legal declarations on functional foods of the four main economic integration organizations in Latin America and the Caribbean (LAC) – Pacific Alliance (PA), Caribbean Community (CARICOM), Southern Common Market (MERCOSUR) and Central American Integration System (SICA) - are based on the Codex Alimentarius system and do not regulate functional foods. The use of Codex in food marketing is an insufficient condition for its application in functional foods. Regulation based on scientific and technology results are required to be used in the economic integration organizations of LAC. Objective. The objective is to analyze the theoretical framework of the legal foundations that could govern the commercialization processes of functional foods, whose research advances have currently only been manifested in nutritional health. This article also seeks to address this gap through a systematic analysis of international regulations. Materials and methods. For this, a review of the literature emanating from two databases from 2018-2023 is carried by applying the legal-economic research method of documentary content analysis, applied to three general food marketing regulations: food safety declarations, regulations for inspections, food manufacturing and food labeling. Results. The results reveal the absence of specific legislation for functional foods in LAC economic integration organizations. Conclusions. The legal principle of marketing based on peremptory norm (also called jus cogens) can be facilitated through side letters, included in the contents of international contracts. along with the registration requirements of industrial property rights of the member countries associations. Arch Latinoam Nutr 2023; 73(4): 297-312.
Keywords: functional foods, Pacific Alliance, Caribbean Community, Southern Common Market, Central American Integration System, food industry.
Introducción. Las declaraciones legales sobre alimentos funcionales de las cuatro principales organizaciones de integración económica de América Latina y el Caribe (ALC) – Alianza del Pacífico (AP), Comunidad del Caribe (CARICOM), Mercado Común del Sur (MERCOSUR) y Sistema de Integración Centroamericana (SICA) - se basan en el sistema del Codex Alimentarius y no regulan los alimentos funcionales. El uso del Codex en la comercialización de alimentos es condición insuficiente para su aplicación en alimentos funcionales. Se requiere que las regulaciones basadas en resultados científicos y tecnológicos sean utilizadas en los organismos de integración económica de ALC. Objetivo. Analizar el marco teórico de los fundamentos legales que podrían regir los procesos de comercialización de alimentos funcionales, cuyos avances en investigación actualmente solo se han manifestado en salud nutricional. Este artículo también busca abordar esta brecha a través de un análisis sistemático de las regulaciones internacionales. Materiales y métodos. Se realiza una revisión de la literatura emanada de dos bases de datos del período 2018-2023 aplicando el método de investigación jurídico- económica de análisis de contenido documental, aplicado a tres normas generales de comercialización de alimentos: declaraciones de seguridad alimentaria, normas para inspecciones, fabricación de alimentos y etiquetado de alimentos. Resultados. Los resultados revelan la ausencia de legislación específica para alimentos funcionales en las asociaciones comerciales de ALC. Conclusiones. El principio jurídico de comercialización basado en norma imperativa (también llamado jus cogens) puede facilitarse a través de cartas complementarias, incluidas en el contenido de los contratos internacionales. junto con los requisitos de registro de los derechos de propiedad industrial de las asociaciones de los países miembros.. Arch Latinoam Nutr 2023; 73(4): 297-312.
Palabras clave: alimentos funcionales, Alianza del Pacífico, Comunidad del Caribe, Mercado Común del Sur, Sistema de Integración Centroamericana, industria alimentaria.
https://doi.org/10.37527/2023.73.4.005
Autor para la correspondencia: Claudia Gómez Gómez, e-mail: [email protected]
The growth and aging of the population, as well as longer life expectancies, make an increase in the demand for functional foods foreseeable in the long term. In the next 50 years, without a doubt, it is expected that the production of these products will become one of the main challenges of public policies, given the need to feed a longer-lived population that will suffer from diseases typical of modernity. such as obesity, osteoporosis, cancer, diabetes, allergies, and dental problems (1).
Additionally, due to climate change and greater industrial development, a lower availability of arable land, water and labor available for agriculture is expected. In this way, a decrease is expected in both the production of cereals and other basic foods in various regions of the globe. Both factors force us to develop technologies that provide sustainability to food production in the long term. Both factors force us to develop technologies that provide sustainability to food production in the long term (2).
While these situations develop in the world, legal regulations evolve according to the political, social, cultural, technological, material, environmental and commercial transformations of each country , Regulation (EU) 1169/2011 of the European Parliament and Council of 25 October 2011 (3) on food information provided to consumers and its variation depends on the purpose of the regulation and the foundations on which it is based, and must be constantly updated, both in the way of presentation of food information and in the fulfillment of its obligations and the clarity of its execution by the concernedparties. Furthermore, regulatory modernization and the use of new technologies can achieve greater security in the Latin American food market, guaranteeing a strengthening of legal security and more adequate protection of the interests of its participants.
This work is a contribution to the commercial debate that affects the regulation of the production and marketing of functional foods in LAC, in which food safety declarations, inspection regulations, and food manufacturing and labeling regulations should be integrated. This type of food is focused not only on nutrition, but also on its application for specific health purposes, using the same biological processes of animals and plants to improve the quality of the final products. Beltrán (4) conceptualizes them as those foods that, in addition to their nutritional value, contain biologically active components that provide some added and beneficial effect on health or that reduce the risk of contracting certain diseases. The concept of “adequate nutrition” is currently transforming into “optimal nutrition” (4), in which functional foods offer the possibility of improving the health of the population and reducing the risk of developing some specific chronic diseases. Among their characteristics is that they must be presented in the form of foods for daily consumption, without producing harmful effects, with nutritional and beneficial properties for the body, as well as the ability to reduce or prevent the risk of contracting diseases, in addition to improving the health status of the individual. Its beneficial effects must be demonstrated in amounts of normal consumption in the diet. Functional foods – which were not identified with this term in the 1920s – do not have a universally accepted definition, but are generally understood as those that provide the body with the necessary amount of vitamins, fats, proteins, carbohydrates and other nutrients necessary for their healthy survival (5) or are known as foods with beneficial effects on human health due to their ingredients or because some harmful characteristic has been removed1. Depending on the country and its legislation, these foods may or may not have a rigorous evaluation with scientific support to be marketed with that designation. In 2020, the global functional foods market was valued at $176,518.97 million and is projected to show a compound annual growth rate of 2.71% during the forecast period 2021-2026 (6) and an average global annual growth of 7.9%. These products represent a specific case, both from an industrial and legal point of view, so, first, the industrial assumptions that surround these products will be analyzed and it will continue by analyzing the legal framework.
The main purpose of this work is to analyze the regulatory progress of functional foods in LAC, taking into account the four main economic integration organizations of the region and their founding countries, which currently do not have regulations on such foods even though their international counterparts’ markets are well appreciated and differentiated. The main associations that we will refer to are the PA (Chile, Colombia, Mexico and Peru), CARICOM (Antigua and Barbuda, Bahamas, Barbados, Belize, Dominica, Grenada, Guyana, Haiti, Jamaica, Montserrat, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname y Trinidad and Tobago), MERCOSUR (Argentina, Uruguay, Paraguay and Brazil)2 y SICA (Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua and Panama).
There are mainly two specific objectives in this work: firstly, it is to identify the legal frameworks in the marketing of functional foods shown in this research and, secondly it is to recognize the challenges of the legal treatment of said products, allowing to make a proposal. The above is in response to the growing needs of a constantly expanding world population with an interest in consuming healthy foods as a promise of better health and quality of life. Then, there arises a problem of collective action that regulates functional foods in a way that is convenient for all the countries that are members of the economic integration organizations and, at the same time, a regulatory problem so that the above-mentioned regulations serve for the adequate marketing of these products and for assertive information to the consumer who prefers to consume these foods.
The work is divided into two parts: the first part reviews the development in the conceptualization and regulation of functional foods and, the second part shows the comparative international regulation showing the challenges present in their commercialization. Finally, a solution to marketing and management is proposed.
From an agro-industrial point of view, covid-19 increased the fragility of the world's food supply chain, leading manufacturers to look for alternative ingredients that overcome trade barriers. This situation creates opportunities for local sellers and regional suppliers of raw materials, if in general, foods have undergone a large number of genetic modifications in order to improve their characteristics. Crops and food products are now physically and genetically modified through biotechnology (7), understood in its traditional or classic sense according to (8) as a set of techniques for manipulating living things or its parts for economic purposes, without direct genetic management; while, in a modern sense it is that set of techniques that use the manipulation and direct genetic transfer of DNA.
The development of new techniques to produce more and better food is an absolute necessity in LAC. Functional foods offer specific health benefits, beyond the regular daily intake of nutrients, controlling cholesterol and improving heart, bone and eye health, among others. This is why the international market has boosted its growth with the fortification of nutritional additives in the offering of its products such as omega-3 fatty acids, fiber, vitamins and minerals.
At the same time, consumers are becoming more health conscious and have begun to pay more attention to their lifestyles and diets, which has also driven its development around the world. However, for them to effectively serve this objective, it is necessary to reevaluate aspects of the information rights they have about the food they consume and avoid practices that may lead to deception.
Facing these challenges being health, ethical, environmental and economic, a change is taking place in the paradigm of what is understood by food. This concept has gone beyond the basic idea of satisfying primary needs, escalating to a new generation of functional foods that seek to fulfill two different aspects: improve health and reduce the risk of contracting diseases.
The history of functional foods dates to 1000 B.C. in China, where there is a long tradition of using certain foods and herbs with healing or therapeutic properties as part of traditional medicine. The term “medicinal food” was used frequently in the literature of the Han dynasty, around 100 B.C.; as well as the “special foods” evidenced in the literature of the Song dynasty of the year 1000 A.D. In the West, the Greek doctor of the 5th-6th century B.C. Hippocrates already had glimpsed the importance of food with his famous phrase “let food be your medicine and medicine be your food” (9), being this philosophy that we can relate to functional foods in the 21st century. In the United States of America (USA), salt with iodine has been used since 1924 to attack goiter disease (10 - 11). Historically and commercially, in markets that consume functional foods it is ensured that they have a beneficial effect on the human organism, beyond the usual nutritional effects and that their consumption improves the health and well-being of those who ingest them, as well as having the possibility of reducing the risk of diseases.
In Japan, since 1930, fermented milk was used to prevent intestinal diseases, by adding the probiotic Lactobacillus casei strain Shirota. Probiotics are understood as “what is provided by live bacteria that are specially cultured and remain active in the intestine” (12). These foods have scientific proof that they strengthen the body's defenses and that is what they sell with an estimated global market value of more than 37 billion dollars, although some gaps remain, and research continues on their effectiveness (13).
The scientific challenge of generating new foods is not new. Biotechnology has been dealing with it for decades. What has changed have been two elements: the pressure for more and better food and the emergence of new production techniques in the biotechnology industry, from which legal regulation cannot remain unrelated. In this industry, different categories can be distinguished, depending on the purpose with which they work. It is from one of them, food biotechnology, that functional foods have emerged. Food biotechnology uses the techniques and processes that use living organisms or their substances to produce or modify food, improve the plants or animals from which they come, as well as develop microorganisms that intervene in their production. The use of these techniques has resulted in positive effects to prevent some specific diseases.
As shown in this work, the entire production process of raw materials that may specifically contain functional foods is very deregulated in LAC.
Since 1999, Brazil is the country with the greatest progress in regulations in LAC, allowing claims that indicate the reduction of disease risk factors. The National Agency for the Supervision of Health (ANVISA) published regulations in 2002. These regulations are in addition to the existing regulations on active ingredients (14). ANVISA intends to convert current indicative instruments into regulations to establish a more rigorous process for approving claims. The guidelines are a non-binding regulatory instrument with the nature of a recommendation. Thus, this research does not include a specific analysis of indicative instruments.
Resolution No. 165 - NTE INEN 2587 on requirements for functional foods in Ecuador (15) is the only one that since 2011 has made official – on a voluntary basis for the LAC region – the minimum requirements that foods must meet for being considered functional. This standard applies to all natural or processed foods that make functional or health claims. However, Ecuador does not belong to any association that is the subject of our study. For its part, in Chile there is no specific chapter of regulations on functional foods and there is no technical body of the State in charge of granting scientific recognition of the content. This differentiation between the regulations of developed and developing countries makes it difficult to commercialize functional foods and reduces the opportunities for national producers in more attractive markets. For this reason, arises the question of the existence of literature that proposes and systematizes advances in standards and public policies that attempt to provide a global legal framework for this type of food, regulate its marketing, understand the implications for the biotechnology industry and protect the consumer. and the environment. The paradigm shift in terms of the conceptualization of food has opened a gap that has been little researched in the world regarding the marketing of these products. While developed economies have implemented commercial and legal practices that seek to protect their production, emerging economies mostly follow the Codex Alimentarius3, which hast been used since 1963 (16), being left at a disadvantage in the food industry regulation. International legislation for functional foods is not homogeneous, which makes regulation difficult in less developed countries where the main raw materials that foods may contain are produced – in a rustic manner and without scientific-technological knowledge of their added value.
For countries that follow the Codex Alimentarius, there are only three types of health declaration (17) a) Functional nutrient declaration; b) Function enhancer declaration, and c) Disease risk statement. These statements must be presented to the consumer as non-preventing diseases. Unlike Japanese legislation, the Codex Alimentarius provides international guidelines on food labeling to avoid misleading consumers into believing that there is exact knowledge of what people should eat in order to remain healthy. Nutrition labeling should not deliberately imply that foods presented with such a label necessarily have any nutritional advantage over those without such labeling.
On the other hand, in European Union (EU) For its part, in the European Union (EU) the project of Functional Food Science in Europe (18) was born, coordinated by the International Life Sciences Institute (ILSI) Europe, which establishes a scientific approach to nutrition and food science. Since 2006’s Regulation (EC) no. 192472006, the EU established definitions, specific criteria and conditions of use regarding nutritional declarations and health properties of foods. Additionally, in USA the regulation of functional foods falls under the Food and Drug Administration (FDA), that allows three forms of marketing —fortified, enriched and improved — and the ILSI of USA - Canada includes modified and non-modified foods in its definition. Finally, Canadian legislation allows therapeutic claims that declare disease risks, as long as they comply with standards and regulations that have scientific support.
Notwithstanding the above, the vision of international law allows us to encompass the commercialization of functional foods within the generic concept of food, adding a greater commercial value to them and consequently appreciating a legal differentiation in the management of intellectual protection and the commercialization of raw materials protected by the patronization of international free trade agreements in LAC. In this context, the following questions arises: Where is the progress in the regulatory framework for functional foods in LAC established? The natural equity of international law exists between the interests of producers with different regulations, and the marketing needs of products that may be considered as raw materials in LAC. It is necessary to propose regulation by country or by associations to advance? This work addresses the existing knowledge gap based on the need to generate valid and reliable information about these foods and their marketing in Latin America and the Caribbean.
Given the need to protect consumers and safeguard the health of the population, in recent decades different models have emerged aimed at providing a general regulatory framework for the food industry. Due to the heterogeneity of regulations, on 20 May 2020, the European Union adopted a harmonized front-of-pack nutrition labeling scheme with the “farm to fork” strategy for a fair, healthy and environmentally friendly food system (19) which combined the essential features of the Nutriscore and Nutrinform systems. In this way, European consumers were allowed to decide which scheme they prefer and forced producers to use nutritional labeling on the front of the packaging and to choose which label to use, complying with requirements established by the regulation on food information provided to the consumer.
On the other hand, Latin American legal systems have opted for a labeling alert system. Within the regulations there are differences with the existence of three models: The Chilean, the Ecuadorian (20) and the European—due to British influence— in the Caribbean. The Ecuadorian model, in its norm NTE INEN 1334-3: 2011, already includes the labeling of food products for human consumption, part 3, requirements for nutrition and health claims. However, Ecuador does not belong to any of the four main economic integration organizations in this study.
Regulation through the Codex Alimentarius has been positively evaluated in terms of health, but it does not regulate complex products such as functional foods, in which the legislation on the sale of food products and their content is not specific. Nor is it responsible for other relevant legal aspects such as intellectual property rights and biosafety regulations that internationally influence the management of biological research and its commercialization as regional foods. (21). According to the above, the provisions of customary international law jus cogens show us the way for change in the regulation on the marketing of functional products given the need to provide the world with high quality food to the population, allowing to differentiate the food product as such from its protection covered by intellectual property. The measure of legal protection available in intellectual property also includes plant varieties with the complexities of regulation in each associated country in the region. For example, in Chile the protection of plant varieties is found in Article No. 19.342, from 1994, a special sui generis legal regime that broadly follows the International Union for the Protection of New Varieties of Plants (UPOV), which was created by the International Convention for the Protection of New Varieties of Plants adopted in Paris in 1961, reviewed in 1972, 1978 and 1991 and which regulates breeder's rights in a similar way to other intellectual or industrial property institutions.
Regarding the routes of definition of foods in the world of the development of technological innovation and the growing demand for healthy foods for human beings (22), the possibility of satisfying it is offered through the products offer from the agricultural and food industry, with an enormous business opportunity for functional foods (17). This is the meaning of understanding that this type of product is an improved food, like a kind of specialized food, but food nonetheless.
Among functional foods there are other types of food characterizations such as probiotics and prebiotics, the latter defined as “a selectively fermented ingredient, or a fiber that allows specific changes, both in the composition and activity of the gastrointestinal microflora, that confer benefits to the well-being and health of the host” and symbiotics, which is the “combination of probiotics and prebiotics” (8). In the 1950s, the WHO established fortified food programs to fight malnutrition, through e-Library of Evidence for Nutrition Actions (eLENA), which can identify evidence-based guidelines for the growing number of global nutrition interventions (23).
It was not until 1990 that Japan became a pioneer in the legislation of these foods, establishing a system for their approval based on health research results and issuing a decree by the Ministry of Health and Welfare. The Japanese food approach (24) is based on a new legal system that regulates them under the large segment of “healthy foods.” — that reach a share of the total internal market of 65%—. In 1993, Shiseido was the first company authorized to sell physiologically functional food by Japan’s Ministry of Health, Work and Nutrition (25), marketing a rice whose globulin protein had been extracted to allow its consumption by allergic people.
What are considered functional foods in Japan are the only type of food product - although not an ingredient - that can legally carry health claims and are composed of functional ingredients that affect the structure and physiological functions of the body. They are considered an example of consumption to control specific conditions such as gastrointestinal health and blood pressure (26). Japanese food legislation has the general designation term kenko-shokuhin, which refers to foods recognized as having health properties, and keyno-sei-shokuhin, for functional foods; FOSHU foods Food for Specified Health Use) currently being used within the legislation (17- 9). Which leads to difficult regulation at the international level. Currently, attempts are being made to issue regulatory documents, minutes and conferences on procedures to regulate functional foods (See [Table 1]).
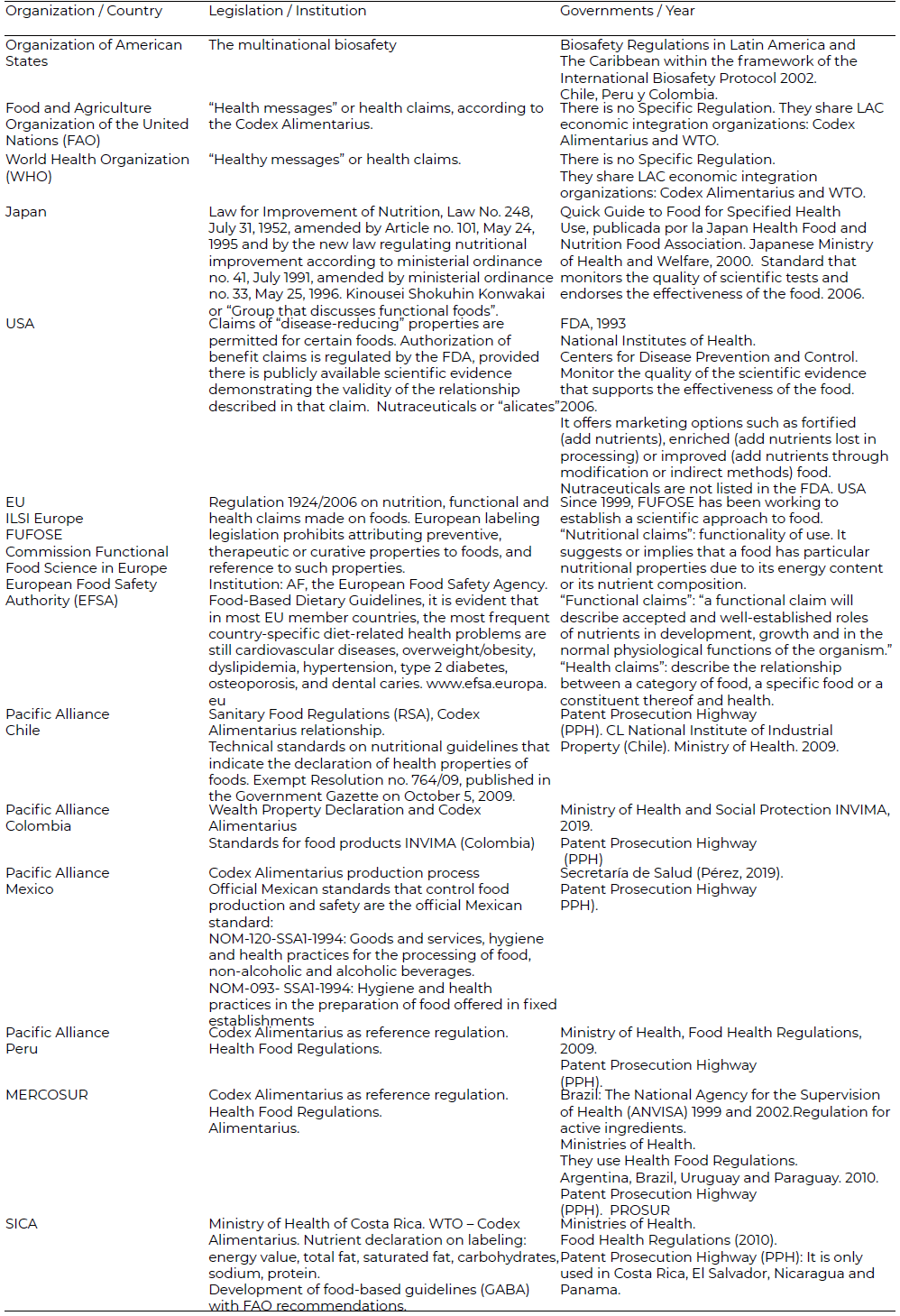
It is therefore pertinent for the countries of the main economic integration organizations of LAC to consider functional foods as improved or specialized, based on the historical justification of international law jus cogens, since they are regulated under the universal spectrum of foods and according to the law. In their international marketing, they should comply with the common requirements of “products”, and their manufacturers may freely market them as such foods, without prejudice to the use and strengthening of side letters — bilateral arrangements for the specific understanding of a particular aspect of an international treaty between two or more states (27) — and the need to clarify the terms of international contracts on plant varieties and industrial property registration of the member countries of the main Latin American economic integration organizations, used in case the food product must first be protected by the recognition of special treatment as a functional food in its origin country.
In conclusion, we observe two legal routes for these products to enter the international market: the first, as common or general foods - which is the normal way in which they are being marketed -; and the second, via inventions protected by industrial patenting or plant variety of international recognition. We observe that the recognition of functional products does not prevent their entry as such into the market of the main economic integration organizations in LAC; either via patent, brand or plant variety from a country outside the association or as new products generated and developed by producers of the commercial block. In this way, competition based on jus cogens is opened and guaranteed against the restrictions of differentiation “by producer”, but not by “product”.
The way to approach the object of study is through the scientific methodology of legal-economic documentary analysis of synthetic content, which brings the law closer to social science in a qualitative theoretical-conceptual way, based on the legislation on health regulations in the foods and evidencing intrinsic and extrinsic relationships to establish a relational framework for the research. The study was applied to three general food marketing regulations: food safety declarations, inspection regulations, and food manufacturing and labeling. For this purpose, the documentary research proposal proposed by (28) and (29) was used. The first authors propose a qualitative methodology- bibliographic documentary research- that can be applied in four phases: to select and analyze documents in order to present coherent data; to use logical procedures (analysis, synthesis); to carry out a process of generalized scientific abstraction on the basis of the fundamental; and to use different data localization techniques, document and content analysis with the procedures of document-type source analysis (review of bibliographic, newspaper and electronic documents sources along with the organization of the information). The second methodology – literature review- is applied based on a review strategy that adheres to the basic principles of systematic reviews with rigor and transparency, while allowing flexible and user-friendly handling of specialized analysis and retrieval methods. The design of the in-depth qualitative analysis consists of eight steps, which are explained below, and a subdivision into three stages in step five: the search for academic literature, snowballing, and capturing gray literature.
In the first step, the feasible research question is established, thinking carefully about both its approach and its formulation and specific language. To formulate the question, the following guidelines were taken into account: that it be a single one, that it be as specific as possible and that it be posed in terms of population, intervention, comparison, outcome and settings (PICOS). In this way, we came to the following research question: What regulatory advances exist in the commercialization of functional foods in the main economic integration organizations of LAC from 2018 to 2023, in comparison with the existing international legislation in North America, Asia and Europe?
The second step corresponds to the implementation of the protocol and explains the research question, according to the aforementioned Hagen-Zanker and Mallett methodology. Thus, the inclusion and exclusion criteria were determined, and the literature generated by the LAC countries that make up the economic integration organizations mentioned above was focused. The exploration carried out seeks to make a synthesis of the main considerations of epistemological influences in the different cultural contexts of the types of functional food, in order to ensure that cultural differences do not affect the research. The analysis scientific review of the literature of articles concerning the topic was carried out in Spanish, English and Portuguese, in the legislation of the LAC countries and internationally. Respecting the list and search strings, Mendeley referencing software was used to manage references from databases, journals and websites. Likewise, citations were imported according to the keyword search results of each database, in order to more easily handle the literary material. Two online databases were consulted—Scielo and Google Scholar— and articles published from 2018 to 2023 were searched under the area of Social Sciences, Law and Health, which contained information on relevant regulatory advances in functional foods, both in the title and in the complete article and integrating the regulations of declarations of food safety, inspections and food manufacturing and labeling. Secondarily, government policies were searched in the four economic integration organizations covered by the study. Likewise, (See [Table 2]) shows the search equations used, in order to complement the study.
Thedataisanalyzedandpresentednarratively with the most important information and is characterized according to content (30). The research reviewers were responsible for compiling the narrative synthesis and reading the material associated with the keywords, which included the articles found in the three mentioned areas from 2018 to 2023.
Regarding the inclusion and exclusion criteria, a critical legal-economic review of the literature was carried out that included the exploration and mapping of the literature, distinguishing the characteristics that differentiate the system from dietary trends. It was divided into three dimensions: regulations of food safety declarations and government policies, regulations of declarations and inspections by product/ market linked as raw materials and, finally, regulations of declarations that affect food manufacturing and labeling. Likewise, a chain was made with the words and expressions “functional foods”, “regulations”, “food safety” and “government policies” with each of the main economic integration organizations in Latin America in [Table 2].
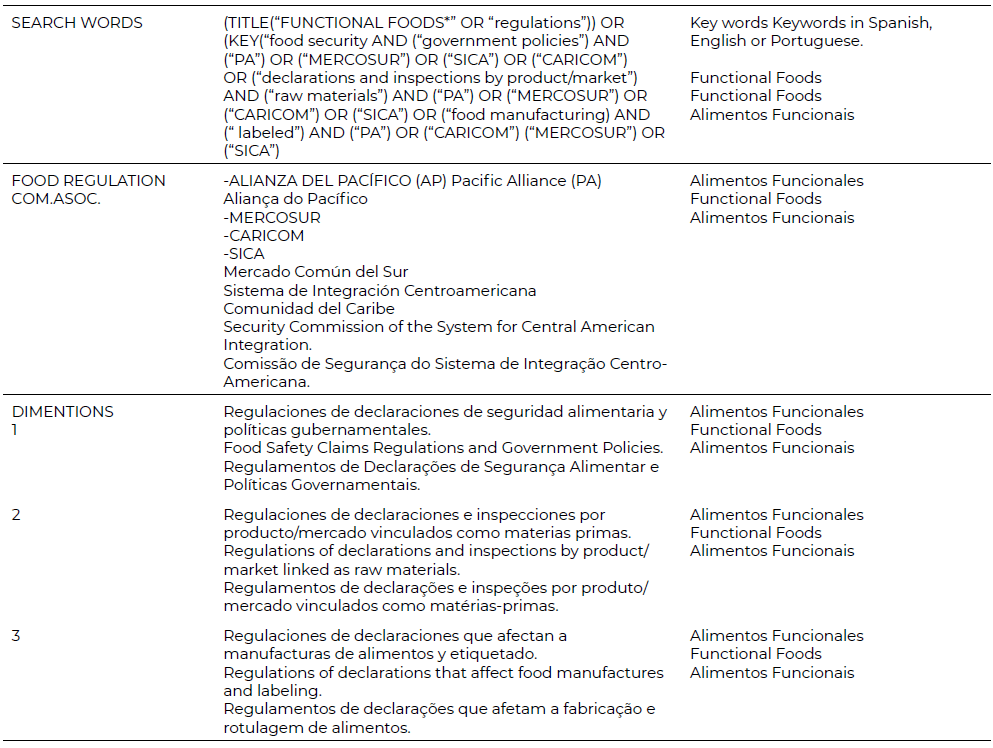
The information was obtained by analyzing the search results of academic literature in the Google Academic and Scielo databases, introducing the search equations with their dimensions and identifying potentially relevant material. The retrieval mechanism consists of three separate but interrelated pathways: academic literature search, snowballing, and gray literature capture. Regarding the search for academic literature, the two databases selected were relevant given their quality, their multidisciplinary nature, open and free access, and they cover publications published in Latin America. Google Scholar is a search engine focused on scientific-academic content and bibliography that indexes publishers, libraries, repositories and bibliographic databases, among others, meeting technical requirements for its dissemination. As well as, Scielo is an online, multidisciplinary scientific electronic library that contains the largest specialized research data in Latin America and that adopts the best practices and international standards for data and research management. These journals are published nationally and communicate basic and applied research of national and foreign authorship, multilingual and that maintain collaborative network work with scientific quality, aligned with the state of the art according to the principles of findability, accessibility, interoperability and reuse (FAIR), as well as diversity, equity, inclusion and accessibility (DEIA). Regardless of whether the journal is legal, economic or social science, it is checked that the search equations exist. To avoid search duplications, research is carried out on the official websites of economic integration organizations. On the other hand, no limits were established on the number of studies to be reviewed because the search did not yield an excessively high number of results.
Furthermore, there were included five reviewers specializing in the subject, requesting five key publications on functional foods with the requested dimensions and publications on functional food regulations were identified as a starting point. The reference lists of these publications were consulted and other relevant publications on the issue under investigation were searched. Finally, the capture of gray literature was carried out by reviewing the relevant material that was intended to be found outside the orthodox peer review channels.
Regarding screening, the publication data and abstracts were downloaded through the Mendeley data reference and, in the second round of screening, the full text of the documents was obtained using the same inclusion criteria.
Regarding the classification of the studies found, the year of publication, geographical coverage and result are mentioned. The quality of the research is evaluated based on national and international sources in assessing the overall robustness of the research corpus. The GRADE system adjusted to the social sciences is used, classifying the results using a nine-point scale in which 7 to 9 is totally related and important, 4 to 6 not so related, and 1 to 3 not very important and not related.
The analysis describes, summarizes and synthesizes the results with the aim of answering the general research question, focusing attention on the included studies. The narrative synthesis describes and compares the results with the intention of showing the evidence base in quantity and quality, and determining the reliability of the review's conclusions. In this way, one can draw one's own conclusions about the strength of the evidence based on relevant information. The methodological strategy used sought the food pattern and provided three schematized qualitative codes. Likewise, it was specifically applied to the legislative and regulatory area, in order to improve the contributions for its narrative synthesis. It was only a methodological option to facilitate the approach to the study and optimize resources in the analysis of the literature.
By analyzing the macroenvironmental context in its legal or regulatory phase, the literature recorded specifically from the perspective of the food area is explored. In this way, it is discovered that most of it focuses almost entirely on nutrition, and not on the legal aspect of it. Likewise, as a search strategy, the terms and contents of the strategic selection of keywords and their combinations were discussed so that it would give us a better scope of the complexity of the topic (31 - 32) and It is also discovered that in LAC there is no documentation. This is even though, preliminarily, the Scielo and Google Academic databases had been selected given that they compile the majority of formal articles published in Latin America and the Caribbean in Spanish, English and Portuguese, and it was expected to find complementary literature or different from the Codex Alimentarius on the advancement of functional food regulations.
According to the proposed conceptual framework, the basic regulations for the commercialization of functional foods were recorded, determining the regulatory specifications and paying attention to each of them, as well as their development by country and as a whole, their interactions. To maintain methodological consistency during the citations and management of the process, the aforementioned criteria were applied, taking into account that the contributions on regulations were broad and reading not only the summaries but also the scope of the content of the articles. Thus, in the investigation of the concept of functional and related foods, it is identified that they continue to be the object of study and that in order to legislate them it is necessary to define them. In that sense, not all countries in the world agree that there is a single definition and LAC economic integration organizations have not conceptualized or regulated them differently either. On the other hand, in other countries of the world food regulations have been modified, according to technological advances.
The information found indicates that not all countries are willing to accept them and little or nothing has been published about regulatory progress in the region in order to contribute to their correct commercialization. From LAC literature, there was found material regarding functional foods focused on their conceptualization, innovation process and applications (33- 42).
Among the main results, it is observed that the articles on the topic suffer from serious limitations—both in Scielo and Google Academic magazines—mainly because a similar approach is not shown regarding the legislative aspect or its relationship with public policy for the type of food. They mostly focus on foods in general or in the area of health based on their general nutritional characteristics. Therefore, the reviews of both databases do not allow obtaining combined information from the three search criteria established in all cases and the information on the marketing of functional foods and foods in general—as raw materials—is mixed without differentiation, nor added value. In both databases, the literature reviews show that they do not consider the member countries of the economic integration organizations studied or all the levels of comparative progress between their similar products, nor do they share information on their legislative and marketing management. What they do share individually as a country and collectively as a trade association is the use of the Codex Alimentarius.
The first results of the analysis in the Scielo database of the areas of social sciences, health and law are shown in (See [Table 3]). The regulations regarding the type of foods addressed have more to do with the quality controls of the products, as well as their production certifications for their commercialization—such as ISO 9000 or HACCP— more than with the identification of its contents, nutrients or special or differentiating characteristics.
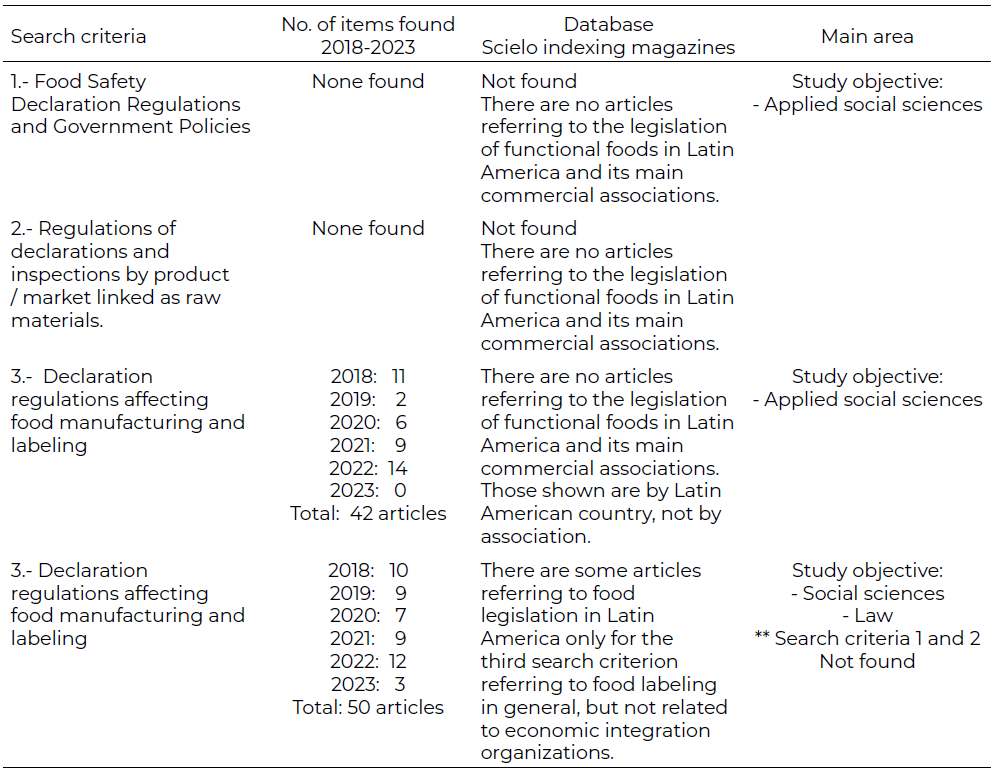
Regarding the Google Scholar database, there is a greater number of documents without arbitration for publication. A collectively and individually research was carried out in MERCOSUR, CARICOM and SICA based on the three search criteria mentioned and no information was found in papers referring to legislative regulations on functional foods in any social, health or law area.
When trying to analyze the information through the regulatory system, an inclination towards the management and certification of functional foods is distinguished internationally, but no evidence is found in LAC of specific characteristics in regulations that support valorization by content or special intrinsic characteristics with added value.
Most reviews agree that the PA, CARICOM, MERCOSUR and SICA cover only general food safety regulations and differentiated government policy. In the consulted Scielo database, there were identified no regulations for food safety declarations and government policies, no regulations for declarations and inspections by product/market linked to raw materials and 42 regulations for declarations that affect food manufacturing and labeling. The articles identified in the Scielo database were found according to the search criteria referring to the regulations explored, as well as the countries from which they arise, the authors of the material, their identification for search verification and the year of preparation. This criterion was carried out in each of the databases consulted. Regarding Google Scholar, the results found were
0 regulations for food safety declarations and government policies, 0 regulations for declarations and inspections by product/market linked as raw materials and 50 referring to labeling related to other topics and not related to LAC economic integration organizations in [table 3].
The effort of international organizations and developed countries to legislate based on the progress in the commercialization of functional foods is clear. For their part, the member countries of the PA, CARICOM, MERCOSUR and SICA do not have special regulations for this type of food and follow that of the Codex Alimentarius proposed by the WHO, the FAO and the World Trade Organization (WTO). Developed countries such as Japan, the US and the EU handle their own approaches. The majority of contributions from the PA countries to literature are from Mexico, Chile, Colombia and, to a lesser extent, Peru. In MERCOSUR, the member countries that publish the most on the subject are Argentina and Brazil, however, there are no indexed articles or formal documentary material predominant in their legislation or internal public policies that show any progress regarding the regulations of USA, Asia and Europe.
Thus, we find an enormous shortage of material in the legislative area of this type of products, both in LAC and in the rest of the world in terms of agreements for their control and commercialization. A greater amount of material was found in the area of human nutrition, which is not the specific object of this study.
It should be noted that in the first analysis of regulation of food safety declarations and government policies, specific public policies and national regulations appear in PA countries, which have a better position on the issue. From Mercosur, Brazil and Argentina present articles on the subject and, from SICA, there are articles on market opening and health policies; CARICOM does not have indexed articles on the commercialization of functional foods.
The second analysis of literature is on the variables of declarations and inspections by product/market linked to the country and the products marketed as raw materials, considering that several goods are similar in Latin America and are marketed differently for national and international markets. The third criterion on claims that affect food manufacturing and labeling was the only one related to labeling regulations, but not related to economic integration organizations.
Functional foods have strongly entered global markets, rapidly gaining market share as value- added products. The term “functional foods” lacks a common definition, but they are thought of as products that offer health, wellness or performance benefits beyond their usual nutritional value. Some are naturally functional, such as high-fiber cereals. Functional foods have become food categories that should be considered within public health policies and have specific legal regulation. As the various functional foods categories, whether in natural or added form, are estimated to exceed the size of the global organic food market and offer potential for new economic opportunities.
The interest of the consumer and the population in general in obtaining optimal diets to maintain good health and prolong the years of life has led to an increase in the demand for natural foods, among which functional foods have had priority. The marketing of “improved” or “specialized” foods is treated as generic under international law, given that its elements constitute substantively the same food substances known since Antiquity. The granting or granting of trademarks or industrial patents to certain food “products” provides them with inventive protection and industrial development as products of the area, which allows for the opening of free competition in international markets, in accordance with provisions of domestic law. The path for LAC countries to develop as food powers depends on the development of legislation that integrates technological platforms that support the production and marketing of functional foods, allowing them, through its regulations and standards, its evaluation and control as well as the improvement of its offer with added value, according to the needs and requirements of the final consumer and, in this way, actively inserting itself not only as an exporter of raw materials, but including research into high-value products of excellence.
A legal and commercial opening is appreciated to accelerate the inclusion of the circulation of improved food products through the benefit of the use of side letters that grant flexibility to the parties to negotiate when the nature of the obligations assumed is asymmetric and that could be managed within the bilateral agreements between the members of the PA, CARICOM, MERCOSUR, SICA and other LAC countries. This would constitute a significant advance for Latin American economic integration organizations in the free circulation of products and the recognition of differentiated functional foods, within which could be consolidated in the future through differentiated foods and, therefore, of greater commercial value. On the other hand, the possibility of improving the marketing position of functional foods is visible both with the granting of trademarks or invention patents and with the recognition of plant variety, increasing the added value that the territory can infer. Considering functional foods as an improved food species. This sets up a discussion about whether food as raw materials should have a measure of differentiation between a natural, invented product, with superior intrinsic qualities and a common food. It should be noted that it is not the intention of this work to define the measure of differentiation between a natural food, a functional one and a patented one.
Taking into account the overlap that the topic has with respect to the legal swarm that constitutes the internal legislation of each country and its constitutional charters, without prejudice to the above and in matters of international law, the qualification of ius cogens is applied, protecting essential values shared by the international community and legally embodying the moral conscience of international society. Production and industry therefore require regulation that allows them to disseminate the healthy properties of functional foods, facilitate their production and marketing, integrating international standards into the adaptation of national legislation and promoting that producers, marketers and consumers have more information about these products.
Most reviews agree that Latin American countries cover only standardized global general food safety regulations—related to nutrition—and differentiated government policies. There is no progress in the regulation of functional foods in the PA, CARICOM, MERCOSUR and SICA trade blocs, with respect to differentiating trade and legislative policies, which distinguishes them from other producers in national or international markets. Therefore, through the strengthening of side letters, bilateral arrangements can be established for specific understanding on functional foods with developed countries.
Likewise, after analyzing the different internal legislations of each country and what is established in its international conventions, there is no express regulation on the marketing of functional foods. Notwithstanding the above, the lack of regulation means that the law applicable to the marketing of these products is protected and protected by the jus cogens of international law, which could operate as a right in the absence of tacit and explicit regulation. Faced with the multiplicity of meanings and global meanings that currently exist in the marketing of food products, truthful information is necessary for the human population and the guarantee of commercial rights innate to international law.
We appreciate the financial support provided by the Catholic University of Temuco for the publication of this article. We also thank the experts who validated the relevance and qualitative content of this material.
The authors declare they have no financial interests or personal relationships that could have influenced the work presented in this article. The authors have no conflicts of interest to disclose.
Publicado: 07/03/2024
Recibido: 13/11/2023
Aceptado: 30/01/2023
1 It is clarified that not in all countries the characteristics of functional foods are scientifically proven, certified and mandatory labeled.
2 Even though Venezuela is a state party, it is excluded by being suspended.
3 The Codex Alimentarius is a set of rules, guidelines and codes of practice approved by the Codex Alimentarius Commission (CAC) and constitutes the main element of the Joint Program FAO/WHO on Food Standards established by FAO and the World Health Organization (WHO) with the purpose of protecting the health of consumers and promoting fair practices in the food trade.